ANTIBODY IDENTIFICATION
Antibody identification or antibody differentiation - in lab slang "Diff".
Both names mean the same and can be used for the test. Since it is impossible to tell from the screening test alone against which antigen an antibody is directed or whether it is perhaps a mixture of antibodies, a positive antibody screening test must be followed by antibody differentiation. The aim is to specify the irregular antibodies found in the search test. For this, the same method is used as for the antibody search test - except that now 8-16 cells are used. Commercially available antibody differentiation panels are available. There is no regulation on the number of cells to be tested against. But in the case of a confirmed antibody (or multiple antibodies), all other antibody specificities must be excluded.
The test cells are in a stabilising medium ready to use.
Each test vial corresponds to a donor whose cells have a specific and known antigen profile.
On the evaluation sheet, each line corresponds to one donor.

How does the procedure work?
One drop from each vial is added to one well of a gel card using the integrated pipette. Then, the same as in the antibody test, a drop of patient serum is added and the gel cards are incubated at 37°C for 15 minutes. The gel cards are then centrifuged and the reaction pattern for each cell is entered into an evaluation sheet. The evaluation sheets are supplied with each new batch of test cells and it is very important that no other (e.g. from a previous batch) is used. Each company will try to have as little variation as possible in the antigen pattern from batch to batch. But the red cells are collected from donors and these can be sometimes not available. So when a new batch of test cells is started, it is important to make sure that all the old forms are removed and exchanged for the new ones.
Here is an antibody differentiation and the evaluation sheet for an 11-cell panel.

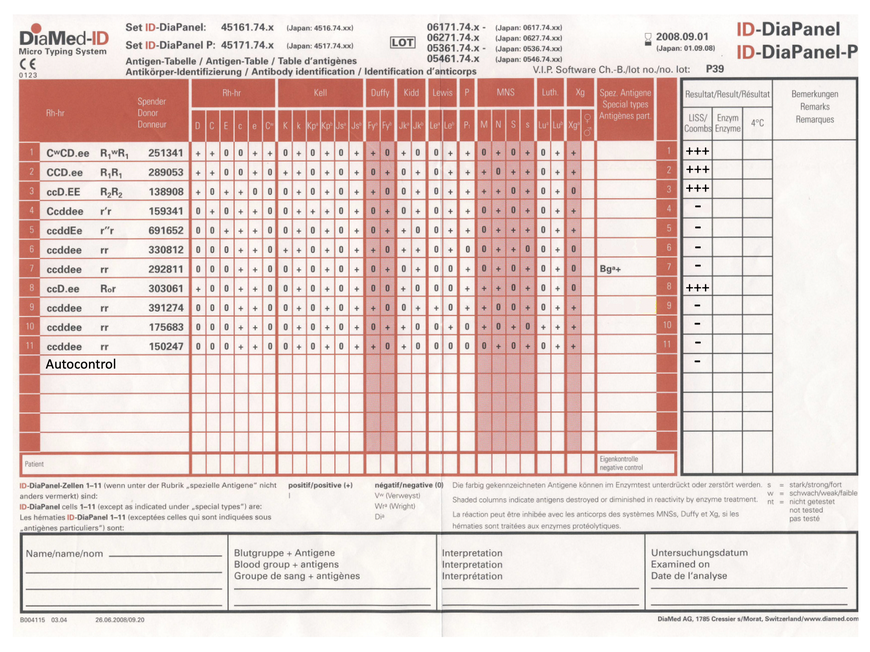
Antibody differentiation is evaluated and interpreted by the exclusion process.
The antibody present appears to be specific. Reactions are seen in only four cells and these are approximately equal in strength, suggesting a single antibody. The exclusion of specificities should only be done using homozygous cells for most antibodies. This is especially important for antibodies that show a dosage effect, such as MNS or Duffy. Only if the antithetic antigen belongs to high-frequency antigens, such as K and k or Lua and Lub, may an antibody be excluded using heterozygous cells. In layman's terms - I can only rule out an antibody if the donor of the test cell has inherited the same antigen from both parents and the other variant is not present at all.
For example: Donor has inherited a Duffy b from mother and father. His cells only express Duffy b on the surface - double dose. Duffy a is not expressed on his cells at all. If this cell does not react with the test serum, I can rule out an antibody against Duffy b. This ensures that even very weak antibodies (with a low titre) are detected by the test.
The approach is as follows: cells 4, 5, 6, 7, 9, 10 and 11 can be used to exclude all antibody specificities except for anti-D. Conversely, all Rhesus D positive cells react strongly positive with the patient's serum.
So it is definitely an anti-D.
Before a specificity is confirmed, it must still be determined whether the patient himself is negative for the antigen. We can only make antibodies against antigens that we do not have ourselves (Landsteiner rule). The formation of auto-antibodies is to be seen as a malfunction of the immune system.
Anti-D is one of the most common antibodies (if not the most common at all). This is mainly due to the immunogenicity of the D antigen. As little as 0.5 ml of blood is enough to trigger a lifelong immune reaction. For that reason, older women who are RhD negative often have an anti-D. They acquired the anti-D during pregnancy or when they gave birth to an RhD-positive child. It can now be avoided by administering Rhesus prophylaxis.
But not every antibody can be differentiated with a single cell panel. Often, not all cells carrying an antigen react equally strongly. Sometimes a reaction can be absent entirely. Then you have to test more cells and use amplification media or enzymes. Especially by using different enzymes, antibodies can often be better differentiated.
An antibody against Duffy a (Fya) will react with a Duffy a positive cell. If you treat this cell with papain, the Duffy antigen is destroyed and the reaction disappears. So if you suspect it might be an anti-Duffy a, another test can be done with the same cells and papain. All the reactions that indicated an antibody to Duffy a should now be gone. Especially Duffy and MNS blood group antigens are sensitive to enzymes.
Many different substances have different effects on blood group antigens. They are particularly helpful when dealing with a mixture of antibodies. In this way, antibody specificities can be "switched off" and are thus easier to separate from each other.
On the evaluation sheets, there are always several columns for reactions. The first one is for LISS/Coombs preparation, and the others are mostly for enzyme and 4°C preparation.
LISS/Coombs means that the cells are suspended in a standardised solution and an anti-human globulin serum (Coombs serum) is added. The procedure is done at 37°C, body temperature. It is considered that antibodies that react under these conditions are also clinically relevant.
It can be questioned to what extent the antibodies always adhere to these rules in vivo. But there is a case report for almost every antibody, with very severe and serious consequences for the patient. And since we do not experiment with patients, in case of doubt any defined antibody will be taken "seriously".
Updated on 03.06.2023.
